The Vibrio cholerae Rapid Test is a rapid visual immunoassay for the qualitative, presumptive detection of Vibrio cholerae O1 and/or O139 in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Vibrio cholerae O1 and/or O139 infection.
PRINCIPLE
The Vibrio cholerae O1/O139 Antigen Rapid Test detects Vibrio cholerae O1/O139 through visual interpretation of color development on the internal strip. The test contain two strip in cassette, in each strip, anti- Vibrio cholerae O1/O139 antibodies are immobilized on the test region of the membrane. During testing, the specimen reacts with anti- Vibrio cholerae O1/O139 antibodies conjugated to colored particles and precoated onto the conjugate pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is sufficient Vibrio cholerae O1/O139 in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
WARNINGS AND PRECAUTIONS
Immunoassay for in vitro diagnostic use only.
Do not use after expiration date.
The test should remain in the sealed pouch until use.
The used test should be discarded according to local regulations.
STORAGE AND STABILITY
The kit should be stored at 2-30°C until the expiry date printed on the sealed pouch.
The test must remain in the sealed pouch until use.
Keep away from direct sunlight, moisture and heat.
Do not freeze.
Care should be taken to protect the components of the kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipment, containers or reagents can lead to false results.
OPERATION
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use. 1. Specimen collection and pre-treatment:
Use the specimens collection container provided in the kit for specimens collection. Follow the operation procedure written on it for instructions. Other clean dry containers could also be used for the same purpose. Best results will be obtained if the assay is performed within 6 hours after collection.
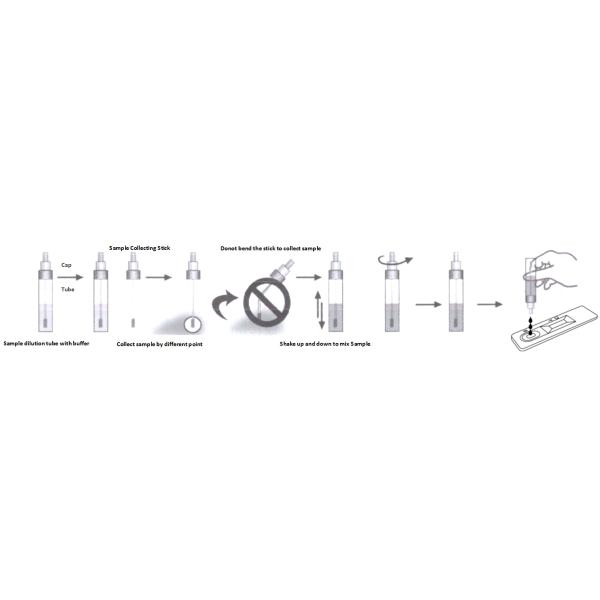
For solid specimens: Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 4 different sites of the feces to collect approximately 50 mg of feces (equivalent to 1/4 of a pea).
For liquid specimens: Hold the pipette vertically, aspirate fecal specimens, and then transfer 2 drops (approximately 50 µL) into the specimen collection tube containing the extraction buffer. Place the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube.
Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if not tested within 1 hour after preparation.
2. Testing
Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed within one hour.
Using a piece of tissue paper, break the tip of the dilution tube. Hold the tube vertically and dispense 2~3 drops of solution into the specimen well (S) of the test cassette.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane. 3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
Note: If the specimen does not migrate (presence of particles), centrifuge the extracted specimens contained in the extraction buffer vial. Collect 80 µL of supernatant, dispense into the specimen well (S) of a new test cassette and start afresh following the instructions mentioned above.