| Sign In | Join Free | My frbiz.com |
|
- Home
- Products
- About Us
- Quality Control
- Contact Us
- Get Quotations
| Sign In | Join Free | My frbiz.com |
|
Brand Name : Dewei
Model Number : DWB0001
Certification : ISO, CE, FDA
Place of Origin : China
MOQ : 5000 units
Price : Negotiable
Payment Terms : T/T, Western Union, L/C
Supply Ability : 200,000 units/Day
Delivery Time : in 20 days
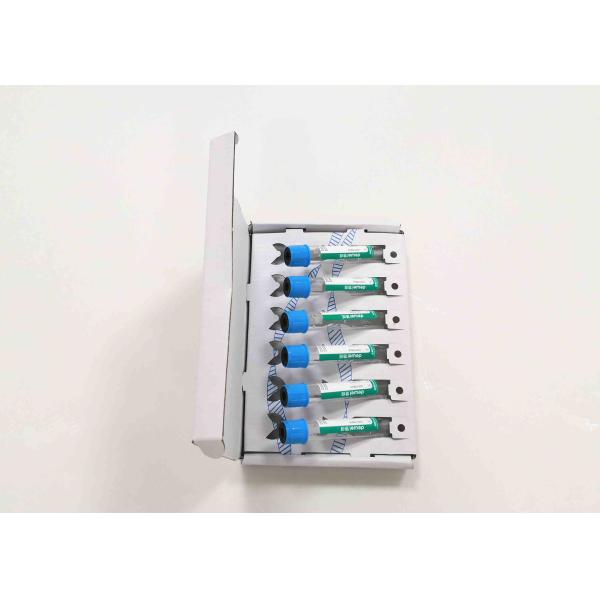
Packaging Details : 10mlx6, 10mlx100
Recyclibg : Disposable
Material : Medical Glass
Temperature : 2-35℃
Sample : Blood
Type : Tube
Shelf Life : 24 months
Properties : Medical Polymer Materials
Sterile Vacuum Tube Cell-free DNA Preservation Blood Collection Tube
【 PACKAGE 】
| Cat No. | Product Description | Package |
| DWB0001 | Cell-Free DNA BCT | 10mlx6 |
| DWB0002 | Cell-Free DNA BCT | 10mlx100 |
【 STORAGE 】
Room Temperature Storage: Store and Transport at 2-35 ℃, can be stable for 7 days after sample collection.
【 APPLICATION】
Wide range of applications: used for NIPT (Non-invasive Prenatal Testing), ctDNA (Circulating Tumor DNA), Cell-free DNA detection of organ transplantation, third-party medical laboratory, hospital laboratory and obstetrics, etc.
Cell-Free DNA BCT protects the Cell-Free DNA in venous blood,thus effectively inhibits nuclease degradation in plasma and DNA release in nucleated cells. By Cell-Free DNA BCT, plasma isolated after centrifugation can be used for molecular diagnosis.
Early pregnancy: The fetal cell-free DNA in plasma is around 0.39% to 11.9% of total free DNA with an average rate 3.4%, the content is very small.
Pregnancy for 12 weeks: The fetal cell-free DNA is 2.33% to 11.4% of total free DNA, with an average rate 6.2%, the content tends to be stable and increasing.
Non-invasive testing requires at least 5% of fetal cell-free DNA. There is a certain proportion of pregnant women who cannot be detected because of low concentration of fetal cell-free DNA, if the protection is not good, even 0.1% of DNA releasing from white blood cells burst can make NIPT results unreliable.


|
|
Cell Free Sterile Vacuum Tube DNA Preservation Medical Polymer FDA Images |